Introduction
Clubroot of brassicas, caused by Plasmodiophora brassicae, is a major soil-borne disease affecting brassica crops, including cabbage, cauliflower, broccoli, radish, and mustard. This disease is particularly problematic for brassica farmers in Nepal due to its persistence in soil and the substantial losses it can cause in crop yield and quality. Brassicas hold an essential place in Nepal’s agriculture as a staple and economically valuable crop, making clubroot a critical issue for farmers.
This post provides a comprehensive overview of Plasmodiophora brassicae, its disease cycle, and effective management practices, with a particular focus on conditions and practices relevant to Nepal.
Taxonomic Classification
| Taxonomic Rank | Classification |
|---|---|
| Domain | Eukaryota |
| Kingdom | Protista |
| Phylum | Cercozoa |
| Class | Phytomyxea |
| Order | Plasmodiophorales |
| Family | Plasmodiophoraceae |
| Genus | Plasmodiophora |
| Species | Plasmodiophora brassicae |
Note: Plasmodiophora brassicae is a protist and not a true fungus, although it exhibits some characteristics similar to plant pathogenic fungi. It is classified under the group Phytomyxea within the Cercozoa phylum.
Symptoms
Initially, infected plants develop pale green to yellowish leaves. As the disease progresses, they begin to wilt during hot, sunny days but may recover at night. Young plants can die shortly after infection, while older plants often survive but become stunted, failing to produce marketable heads.

Source: Gerald Holmes, Strawberry Center, Cal Poly San Luis Obispo, Bugwood.org
The most distinctive symptoms of the disease are found on the roots, which develop spindle-shaped, spherical, knobby, or club-like swellings. These swellings may appear as isolated lumps or merge to cover the entire root system. Older, larger clubbed roots often break down before the season ends due to secondary infections by bacteria and other fungi.

Source: Gerald Holmes, Strawberry Center, Cal Poly San Luis Obispo, Bugwood.org
Disease Cycle
The disease cycle of Plasmodiophora brassicae, the causal agent of clubroot in brassica crops, is complex and includes multiple stages within both the host plant and soil environment. The cycle involves both primary and secondary infections, along with highly resistant spore stages that allow the pathogen to survive in the soil for years. Here’s a detailed breakdown of each stage in the life cycle:
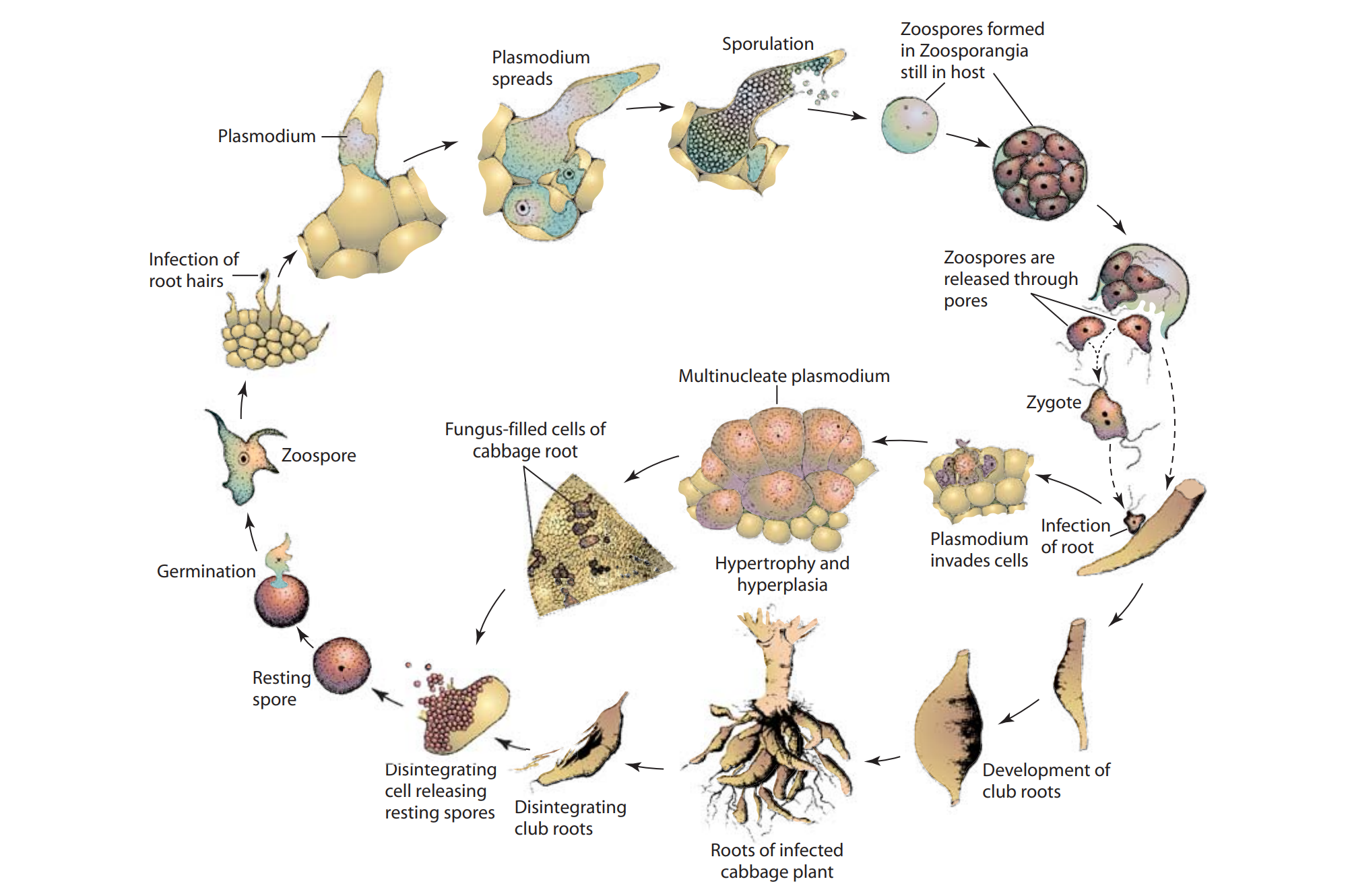
Source: Plant Pathology by George N. Agrios
i. Survival in Soil (Resting Spore Stage)
- Resting Spores: The disease cycle of P. brassicae begins with the formation and long-term survival of resting spores in the soil. Resting spores are thick-walled, spherical structures that allow the pathogen to remain dormant in soil for many years, often up to 20 years, in the absence of a susceptible host.
- Resistance: These spores are extremely resistant to environmental stresses, including desiccation, temperature fluctuations, and microbial degradation. This resistance enables P. brassicae to persist in fields and makes eradication challenging.
- Inoculum Source: Resting spores are the primary inoculum for new infections. Once they sense a suitable environment and the presence of a host plant, they are ready to resume their life cycle.
ii. Germination of Resting Spores and Release of Primary Zoospores
- Environmental Triggers: When environmental conditions are favorable—such as moist soils, mild temperatures (15–25°C), and slightly acidic pH (usually below 7)—resting spores begin to germinate.
- Zoospore Release: The germinated spores release motile, flagellated cells known as primary zoospores. These zoospores are capable of swimming in the thin water films around soil particles, allowing them to move toward plant roots.
- Root Chemotaxis: Zoospores are attracted to the roots of cruciferous plants by root exudates, which are chemical signals released by plant roots. These signals help guide the zoospores to their target, where they can initiate infection.
iii. Primary Infection (Root Hair Infection)
- Attachment and Penetration: Upon reaching the root hairs, the zoospores attach to and penetrate the root hair cells. Once inside, they shed their flagella and transform into primary plasmodia (a multinucleate, amoeboid stage).
- Development of Primary Plasmodium: Within the root hair cells, the primary plasmodium grows by feeding on host cell nutrients, initiating the infection process.
- Secondary Zoospore Formation: The primary plasmodium eventually divides and forms a new generation of motile cells, known as secondary zoospores. These secondary zoospores are released into the soil, where they infect the root cortex and other parts of the root system.
iv. Secondary Infection (Cortical Cell Infection and Gall Formation)
- Secondary Zoospore Penetration: The secondary zoospores reinfect the host roots, this time penetrating the cortical cells and deeper tissues of the root system.
- Formation of Secondary Plasmodium: Inside the cortical cells, the pathogen forms a secondary plasmodium. This stage is highly invasive, as it spreads throughout the root tissue.
- Stimulating Abnormal Cell Growth: The presence of the secondary plasmodium triggers abnormal cell division (hyperplasia) and cell enlargement (hypertrophy) within the root tissue, causing the characteristic club-shaped galls to form. This swelling disrupts root function, impeding water and nutrient uptake.
- Above-Ground Symptoms: These galls cause above-ground symptoms, such as stunted growth, wilting, yellowing leaves (chlorosis), and reduced vigor, due to compromised root function.
v. Formation of New Resting Spores and Dispersal in Soil
- Sporulation: As the season progresses, the secondary plasmodium differentiates into new resting spores within the infected root cells.
- Release into Soil: When infected plant tissues decay at the end of the growing season or upon plant death, millions of newly produced resting spores are released back into the soil, replenishing the soil inoculum and ensuring future cycles of infection.
- Spread of Inoculum: Resting spores are dispersed through soil movement (e.g., via contaminated farm equipment, water runoff, and soil particles carried by wind). In some cases, spores may also be spread by infected seedlings transplanted from contaminated nursery soils.
Environmental and Host Factors Influencing the Disease Cycle
The progression of the clubroot disease cycle is influenced by environmental conditions and host susceptibility:
- Soil pH: Clubroot disease is most severe in acidic soils (pH < 7), as low pH promotes the germination of resting spores and zoospore activity. In contrast, increasing soil pH above 7.2 (usually through lime application) can significantly reduce infection rates.
- Soil Moisture: High soil moisture is essential for zoospore movement and infection. Wet soils or poorly drained fields create favorable conditions for zoospore dispersal and increase the likelihood of root hair infection.
- Soil Temperature: The optimal temperature range for infection is between 18–25°C. At lower or higher temperatures, zoospore activity decreases, reducing infection potential.
- Host Crop Susceptibility: Continuous cultivation of crucifers in the same field increases the risk of clubroot development. The presence of susceptible plants year after year builds up a high concentration of resting spores, intensifying the disease over time.
- Plant Growth Stage: Young plants are particularly vulnerable to severe infection, which can kill seedlings soon after infection. Older plants may survive infection but suffer from stunted growth and reduced marketability.
Disease Management
Effective management of clubroot disease in crucifers, especially in Nepal where the cultivation of crops like cabbage, cauliflower, and radish is common, requires an integrated approach. Clubroot, caused by Plasmodiophora brassicae, can persist in the soil for many years, making it challenging to control. Here are the best and scientifically supported modern management practices for clubroot in Nepal, focusing on cultural, chemical, biological, and genetic strategies:
i. Soil pH Modification (Liming)
- Raising Soil pH: Clubroot is more severe in acidic soils, typically below pH 7. Raising the soil pH to above 7.2 can significantly reduce spore germination and disease severity. Applying lime to the soil is an effective way to achieve this. In Nepal, where acidic soils are common in some regions, this practice can be particularly useful.
- Application Timing: Lime (such as calcium carbonate) should be applied several months before planting to allow time for it to react and raise soil pH effectively.
- Recommended Rate: The amount of lime needed depends on soil composition, but typically 2–5 tons per hectare can be used to raise the pH to the desired level. Regular soil testing is essential to monitor pH changes and determine liming requirements.
ii. Crop Rotation with Non-Host Plants
- Rotating Crops: Clubroot spores can survive in the soil for up to 20 years, but the population density of spores can decrease over time if cruciferous crops are not planted. Rotating with non-host crops, such as cereals (wheat, maize, rice) or legumes (beans, peas), can help reduce inoculum levels in the soil.
- Rotation Duration: A rotation cycle of at least 3–4 years between crucifer crops is ideal, though in heavily infested fields, longer rotations may be necessary.
- Intercropping with Non-Hosts: Intercropping crucifers with non-host crops may also help reduce the disease spread.
iii. Use of Resistant Varieties
- Resistant Cultivars: Breeding programs have developed clubroot-resistant varieties of common crucifer crops. Using resistant varieties reduces the chance of infection and can limit the spread of the pathogen.
- Local Adaptation: Resistant varieties should be adapted to local growing conditions in Nepal. While such varieties may be limited for some crops, research institutions and seed companies are increasingly focused on developing region-specific resistant varieties.
- Avoid Monoculture: Continuous planting of a resistant variety may lead to pathogen adaptation. Rotating different resistant varieties can help prevent this issue.
iv. Soil Amendments and Fertilizers
- Calcium and Magnesium Fertilizers: Alongside liming, applying calcium and magnesium-rich fertilizers can help improve resistance to clubroot by reinforcing root cell walls and reducing soil acidity.
- Organic Amendments: Adding organic matter like compost can improve soil structure and water retention, which can reduce the pathogen’s mobility in soil. However, care must be taken as excessive organic amendments could create overly moist conditions, which are favorable for clubroot.
- Potassium: Increasing potassium levels in the soil has been shown to improve plant tolerance to clubroot in some cases, so using balanced fertilization practices is beneficial.
v. Improving Soil Drainage
- Drainage Management: Since P. brassicae requires water for spore movement and infection, improving soil drainage can help reduce disease incidence. Raised beds, furrow irrigation, and avoiding waterlogged conditions can make the environment less favorable for clubroot.
- Water Management: Avoiding excessive irrigation and ensuring that fields are not overwatered are crucial steps in preventing clubroot infection, especially during the early plant growth stages when roots are most vulnerable.
vi. Soil Solarization
- Using Solarization: In regions with high temperatures and intense sunlight, solarizing soil with clear plastic sheets can help reduce spore levels. This method involves covering the soil with plastic for 4–6 weeks during the hottest part of the year to heat the soil and kill pathogens.
- Effectiveness: Solarization can be effective in Nepal’s warmer lowland regions. However, it may be less practical in cooler, higher-altitude areas.
vii. Biological Control
- Biocontrol Agents: The use of biocontrol agents like Trichoderma harzianum and Bacillus subtilis can help suppress P. brassicae in the soil. These beneficial microbes compete with the pathogen or produce substances that inhibit its growth.
- Application Methods: These agents can be applied as seed coatings, root dips, or soil drenches to increase their effectiveness. Studies have shown that these agents can reduce clubroot severity and are an environmentally friendly alternative to chemical treatments.
- Local Adaptation: Using biocontrol agents adapted to Nepalese soil conditions can improve their effectiveness, so locally available strains should be prioritized.
viii. Chemical Control
- Soil Fumigants: In extreme cases, soil fumigants like metam sodium or dazomet can be used, but they are costly and harmful to the environment. These chemicals should only be applied as a last resort and in accordance with safety regulations.
- Pre-Plant Fungicides: Some pre-plant fungicides, like fluazinam, have shown effectiveness against clubroot. However, they should be used carefully to prevent resistance development and environmental contamination.
- Integrated Approach: Chemical treatments should be integrated with other cultural practices (such as liming and crop rotation) to maximize their effectiveness while minimizing environmental impact.
ix. Sanitation Practices
- Preventing Pathogen Spread: The clubroot pathogen can be spread through contaminated soil, water, and equipment. Thorough cleaning of farming equipment, boots, and tools can prevent introducing or spreading clubroot to unaffected fields.
- Seedling Management: Avoiding infected seedlings from nurseries is essential, as they can introduce P. brassicae into clean fields. Ensuring that transplants come from clubroot-free nurseries is critical.
Integrated Management Summary
For effective clubroot management, farmers in Nepal should adopt an Integrated Disease Management (IDM) approach that combines the methods outlined above. The focus should be on:
- Liming and pH management to make soils less hospitable for P. brassicae.
- Rotating crops with non-hosts to reduce pathogen build-up.
- Using resistant varieties to minimize susceptibility.
- Employing biological agents and balanced fertilization to strengthen plants and suppress pathogens.
These practices, along with strict sanitation measures and soil management, can significantly reduce the incidence and severity of clubroot in cruciferous crops in Nepal. The IDM approach not only helps in managing clubroot sustainably but also ensures better crop yield and profitability for farmers.





